Technological and scientific advancements are consistently pressing forward, offering innovative society solutions to auld dilemmas. Atomic absorption spectroscopy is considered one such development that yielded countless benefits to many industries. Even though this procedure dates back to recent years, current advancements in technology and automated work areas enable scientists to conduct these procedures with enhanced reliability and efficiency.
Many analytical methodologies are available, and picking the most suitable from the best suppliers like https://www.agilent.com/en/product/atomic-spectroscopy is the key to achieving reliable, accurate, and actual-world results. An excellent selection needs a basic understanding since each has its limitations and strengths. Also, it needs a clear understanding of your laboratory’s analytical needs.
The following guide will provide you with an essential insight into the most popular techniques and offer information significant to help you select the one that perfectly suits your particular requirements and applications.
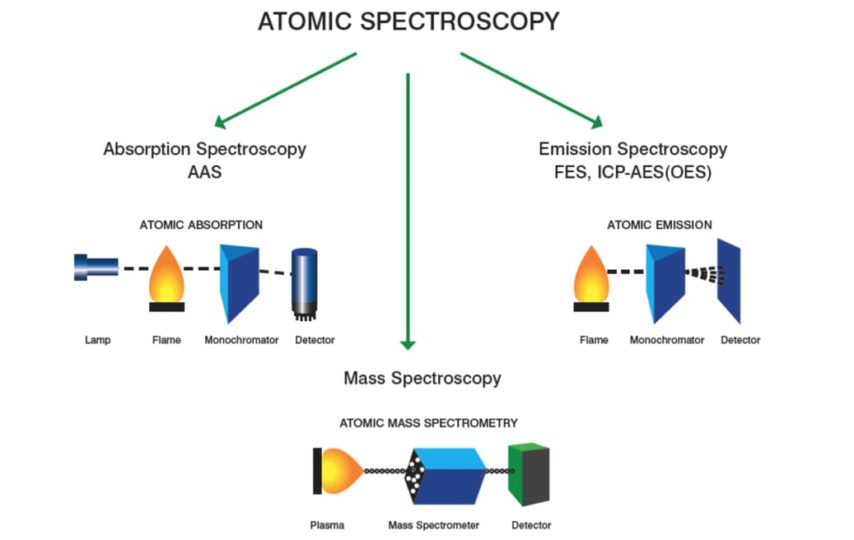
There are three vastly accepted analytical procedures: atomic emission, atomic spectroscopy, and atomic absorption, which will create the topic of our discussion, enabling us to go into the significant depth of the most popular methods used presently, such as:
- Graphite Furnace Atomic Absorption Spectroscopy
- Flame Atomic Absorption Spectroscopy
- Inductively Coupled Plasma Mass Spectrometry
- Inductively Coupled Plasma Optical Emission
- Flame Atomic Absorption Spectroscopy
Atomic absorption happens when a ground state atom absorbs energy in light in a particular wavelength and is measured to an excited state. The quantity of light used at this wavelength will improve as the number of atoms of the prefered element in the light path grows.
The relationship between the concentration of the analytic present and the amount of light absorbed is a known quality that can be used in determining unknown sample concentrations by assessing the amount of light they absorb.
Graphite Furnace Atomic Absorption Spectroscopy
When it comes to graphite furnace atomic absorption spectroscopy, the specimen is introduced straight into a graphite tube, later heating in a structured series of steps to remove the solvent and significant matrix samples and atomize the remaining specimen. Thus, the entire analyte is atomized. However, they will keep the atom in the tube for an extended time. Consequently, detection and sensitivity limits are greatly enhanced over Flame Atomic Absorption.
Graphite analysis takes longer than Flames sampling, and lesser components can be determined using Graphite Flame Atomic Absorption.
Inductively Coupled Plasma Optical Emission Spectroscopy
ICP is argon which is maintained by when ionized argon gas and RF field are interacting. The plasma can get to a maximum temperature of 10,000k, enabling the successful atomization of the elements in a specimen and lessening the prospective chemical interferences.
Inductively Coupled Plasma Mass Spectroscopy
When it comes to (ICP-MS), the argon ICP will generate single charged ions from the component species within a specimen directed into a mass spectrometer and divided based on their mass-to-charge ratios. Ions of the picked mass-to-charge ratio are therefore directed to a sensor determining the number of ions available.
It is fundamental to consider selecting the atomic application and technique instruments from the ideal and recognized firms like agilent.com/en/product/atomic-spectroscopy.
With the availability of various atomic spectroscopy methods, it is also vital for experts in laboratory management to decide which of the instruments is ideally suited to their specific analytical needs.